
A D V A N C E D
M A T E R I A L S
&
P R O C E S S E S |
N O V E M B E R / D E C E M B E R
2 0 1 5
6 3
7
FEATURE
H
ydrogen furnace atmospheres in
conjunction with nitrogen or argon,
carbon monoxide, and methane
provide protective and oxide-reducing
characteristics, which enhance the physi-
cal and chemical properties of heat treated
metal products. Gas mixtures containing
hydrogen are used as a protective atmo-
sphere in many heat treating processes in-
cluding annealing, brazing, and sintering.
This article provides an overview of Hydro-
prime, a unique steam methane reformer
that uses an integrated heat recovery sys-
tem to reduce the cost and improve the re-
liability of hydrogen gas supply.
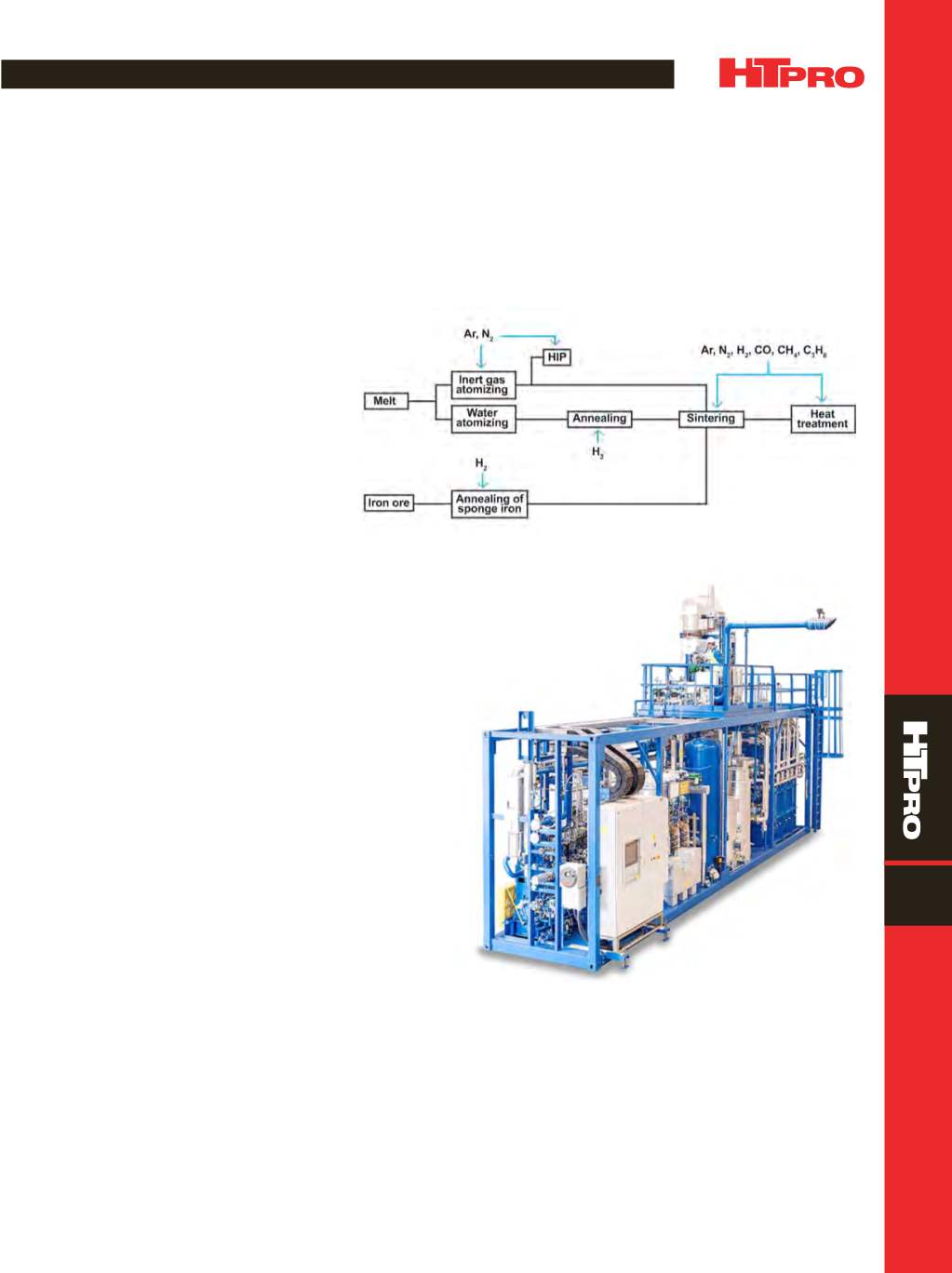
Hydrogen and nitrogen atmospheres are used in the
sintering and annealing of powder metallurgy (PM) parts to
enhance the physical and chemical properties of these prod-
ucts as shown in Fig. 1. Relatively small volumes of hydrogen
(<1095 Nm
3
/h, or 1 MMSCFD, million standard cubic feet per
day) are supplied in bulk by trailer, or produced on site by
electrolysis, methanol, and ammonia dissociation, as well as
steammethane reforming (SMR). Steammethane reforming
is the dominant method used to produce hydrogen at a rela-
tively large scale (>1095 Nm
3
/h), but the technology has not
been widely adopted at a small scale due to cost and reli-
ability considerations. Linde’s Hydroprime plant is an inno-
vative hydrogen generator based on proven steammethane-
reforming technology. The plants are compact, efficient, and
flexible. Figure 2 shows a representative plant.
HYDROPRIME PROCESS TECHNOLOGY
Steammethane reforming is the predominant method
used to produce hydrogen on an industrial scale. Hydro-
prime plants use a unique heat integration concept com-
binedwith SMR. In the process, desulfurized natural-gas feed
is mixed with preheated water and fed into tubes filled with
nickel catalyst. Reactions that occur at elevated temperature
and pressure include:
•
Reforming: CH
4
+ H
2
O
↔
CO + 3H
2
(1)
•
Shift reaction: CO + H
2
O
↔
CO
2
+ H
2
(2)
Approximately 75% of the conversion to hydrogen oc-
curs in reaction (1). Reaction (2) drives the equilibrium bal-
UNIQUE GAS GENERATOR PROVIDES LOW COST,
RELIABLE HYDROGEN SUPPLY
A Hyrdroprime gas generator plant offers a flexible hydrogen supply
solution to the powder metallurgy industry.
Goutam Shahani, Kyle Finley, Nick Onelli, and Grzegorz Moroz,
Linde Group, Blue Bell, Pa.
Fig. 1 —
Gas atmospheres are used in the production of metal powders and in the
sintering and heat treatment of powder metallurgy parts.
Fig. 2 —
Representative Hydroprime plant.
ance further to yield a hydrogen-rich gas. Reforming is an
endothermic reaction, while shift conversion is an exother-
mic reaction. Both reactions (1) and (2) occur in the reformer.
However, only reaction (2) occurs in the shift converter. The
shift reaction uses a promoted iron-oxide catalyst. Both re-
actions are equilibrium limited based on outlet temperature
and pressure. Reaction products are a mixture of H
2
, CO, CO
2
and H
2
O. A simplified block flow diagram of the SMR process
is shown in Fig. 3.


















